Piney Point - Tampa
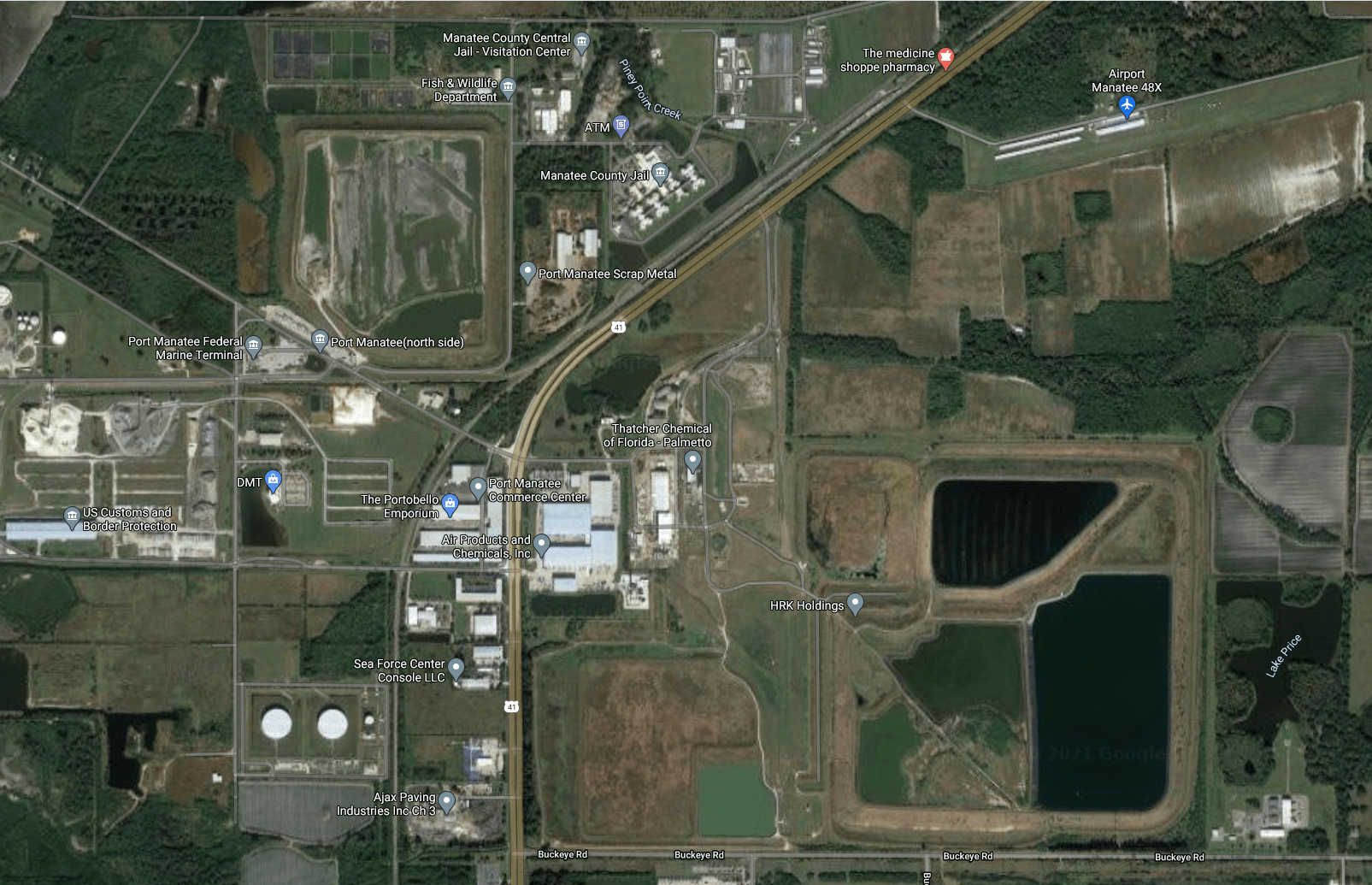
Let's start with a little story about Piney Point, Florida. Way back in 1857 a company started known as Borden. It was a company that specialized in condensed milk, but over time Borden started exploring the production of ink, fertilizer and plastics.
So now in 1966 a phosphate plant opens up in Piney Point Florida to help with fertilizer production. It's job is simple - produce phosphorus to be used in fertilizer. This location is not far from Tampa Bay water and probably under a few minutes drive to hit the shore. It doesn't take long (4 years) until fishers in the area notice fish showing up dead and its discovered that Borden Chemical new phosphate plant is just dumping wastewater into the harbor.
Though what is the wastewater of a phosphate plant? Let's take a step back and learn what happens at a phosphate plant, but first a lesson in phosphate.

Enter phosphate rock - a sedimentary rock that may be between 4% to 20% of phosphorus. A few tricks in the enrichment industry might be able to pull this up to 30%, but for the sake of this blog just understanding phosphate rock is naturally occuring is enough.
Nearly everything in the atmosphere needs phosphorus. So we start this cycle with the weathering of rocks, which breaks them down into little bitty chunks which when paired with rain starts leaking phosphorus into the soil.
- Plants via roots ingests phosphorus from weathered natural rocks
- Plant eating creatures eat plants which now contain phosphorus
- Animal eating creatures eat creatures which contain phosphorus
- Decomposers like mushrooms, worms or mold eat the dead remains which contain phosphorus
- Decomposers release phosphorus as a waste back into soil
This is a natural occurring cycle, but farmers looking to maximize yield on crops may interject into the process with directly applying fertilizer (which contains phosphorus) right to the plants. This might be perfectly fine, but the ecosystem is getting more phosphorus than it can handle.
So a rainstorm comes along and carries all that extra phosphorus towards waterways. Water + phosphorus = algae. Enough algae can block rays of sun from reaching the water - this starves the remaining plants of vital sun and leads to oxygen starvation. This ends up killing the fish. So like most things in life – too much of something is a problem.
So now that we understand phosphorus. How does a chemical plant take absurd amounts of phosphate rock and produce phosphoric acid?
You basically need sulfuric acid first - which requires a ton of sulfur, water, oxygen and heat. This sulfuric acid then can be mixed with the phosphate to extract phosphoric acid. This reaction isn't without by-products though - you'll get:
- phosphogypsum (gypsum) - a low radioactive useless calcium sulfate
- wastewater (high in metals) - unsafe for deposit to water bodies
This wastewater can be reused, but you'll generate more than you can re-use so it will pile up. Sometimes this wastewater is simply dumped on top of lined gypsum stacks to evaporate. Otherwise large retention ponds are produced to hold this liquid - this is with the understanding that these byproducts aren't safe for the environment.
The gypsum stacks are somewhat radioactive due to the naturally occurring uranium and thorium. The EPA for this reason banned the applications of re-using this by-product for anything. So it sits in mounds sometimes holding another dangerous material, until the same organization (EPA) decided to allow it road construction.
Did the late 1900s not yet understand the required safety of phosphate plants? Since the 1966 plant was just dumping the wastewater right into the bay. This led to some issues which ultimately led to Borden giving up the plant to a company specializing in phosphate - AMAX Phosphate.
It must have been an expensive problem to solve, so AMAX gave up the plant and sold it to FCS Energy. This didn't take long either until FCS Energy got absorbed into Consolidated Minerals. Once again you know the tale - this was sold to Royster Phosphates and this is where our story gets interesting.
Royster happened to be the company in charge in 1989 when a storage tank leaked 23,000 gallons of sulfate then a few years later in 1991 had sulfur leaks creating acid clouds that killed 3. [source]
The FBI gets involved for some reason and the plant changes hands again to Mulberry Corporation in 1993. It must be an expensive gig as Mulberry shuts it down in 1999. You may think the story ends here, but unlike nuclear energy that requires strict shutdown plans - the plant was just abandoned in 2001. This happened inline with Mulberry filing for bankruptcy unable to pay for the upkeep of these dangerous by-products.
The EPA (Federal) stepped in for a bit, but then they appointed Florida's Environment Protection agency to oversee it. In 2004 a hurricane caused some damage leading to 70 million gallons of that wastewater to leak.
The current owner (HRK Holdings) bought it in 2006 under the clause to maintain the gypsum and wastewater. Just like clockwork another large leak (170 million gallons) happened a few years later in 2011. This just lead to HRK Holdings filing for bankruptcy.
History about Piney Point companies was pulled from Minerals Yearbook 1988
I'm not happy with HRK though, they sold some of the storage of the reservoirs to take the dredging from a Port Manatee expansion. [source] A dated aging storage of wastewater should not have been a home for dredged material storage and probably led to the leak in 2011.
So now the property sat with rainwater growing the reservoirs until March of 2021 when leaks were detected in the containment walls of a stack of gypsum and wastewater.
It seems this plant has never been properly fixed or managed in over 50 years. There are entire news articles that dive into this history of accidents. What started as a plant with no regard for environment protections just simply passed the buck from company to company to pick up the mess. A sad truth with many things in our world, like the recent news story of 767 tons of DDT barrels dumped into the ocean.
So now we sit in the present time with the state authorizing 400 million gallons of wastewater to be dumped into the bay. I'm no environmental scientist. I have no idea what this will do in the short term or long term, but now I'm getting curious why we have these crazy "red-tides" or algae blooms. Is it the natural ebb and flow of the world or is something pushing the world's hand?
